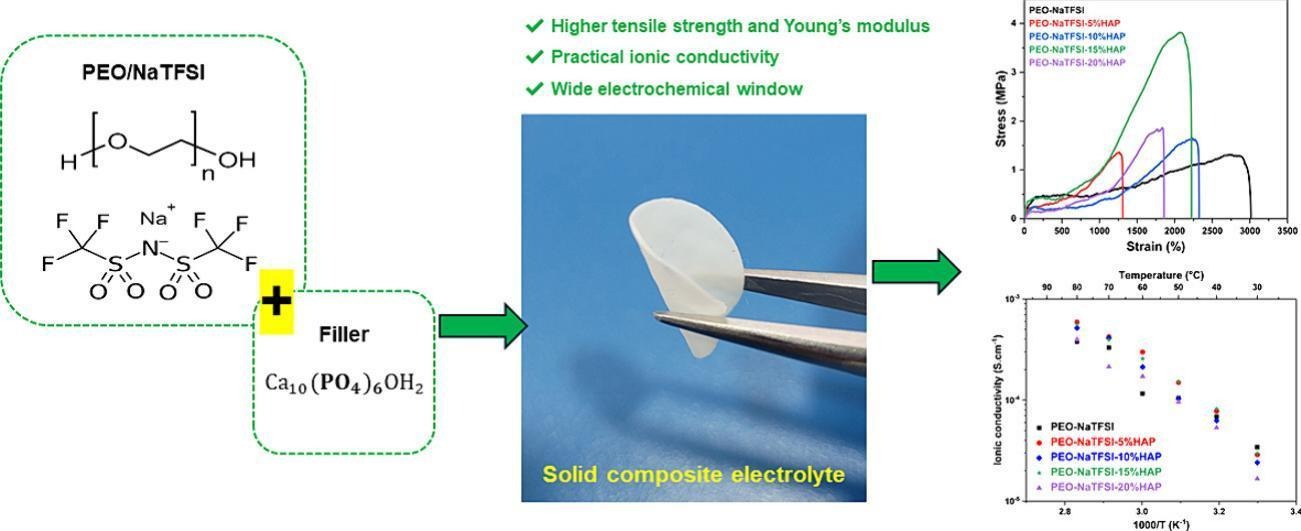
Solid-state sodium-ion batteries have gained notable attention recently. Their appeal stems from the inherent safety feature of solid-state batteries and the abundant availability and low cost of sodium resources. Solid composite electrolytes especially have received considerable interest due to their distinct advantages resulting from the integration of fillers into the solid polymer electrolyte matrix. Herein, we synthesized hydroxyapatite (HAP) by a simple co-precipitation method and used it as a filler to enhance the performance of the solid polymer electrolyte based on polyethylene oxide-salt (PEO-NaTFSI). The incorporation of the HAP filler significantly enhanced the mechanical properties of the PEO-NaTFSI electrolyte, achieving almost threefold improvement. At 70 °C, the composite electrolyte displayed an impressive ionic conductivity of almost 10−4 S/cm and a sodium transference number of 0.38. Additionally, it exhibited a wide electrochemical operating window ranging from 1 to 5.10 V. The composite electrolyte was then evaluated with a recently developed cathode electrode, Na4CrFe(PO4)3, with sodium metal as the anode. The cycling test results demonstrated a specific discharge capacity of 124 mAh/g at C/20 current rate at the fifth cycle. The successful incorporation of HAP as a filler in the solid polymer electrolyte serves as a compelling proof-of-concept for the future development of composite electrolytes based on calcium phosphate fillers. This finding opens new avenues for the development of solid electrolytes towards safer and more efficient sodium-ion batteries.
Publications
From bone tissue to batteries: Hydroxyapatite as a filler to enhance the mechanical, thermal, and electrochemical properties of electrolytes for solid-state sodium-ion batteries,